News
Why does the capacity of lithium-ion batteries decrease?
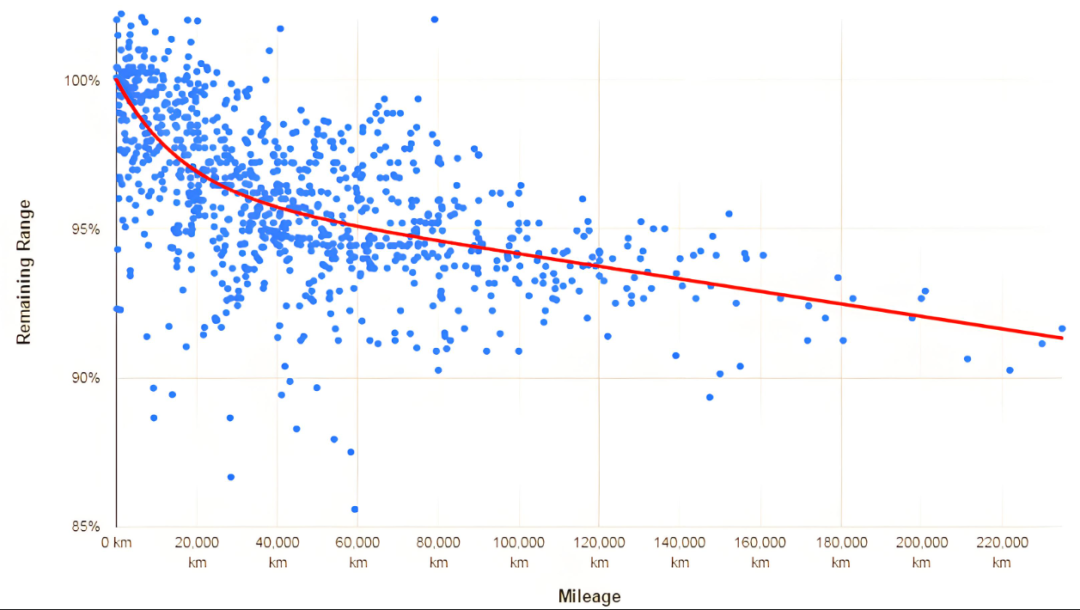
Lithium-ion battery capacity decay refers to the phenomenon that lithium-ion batteries gradually lose their available capacity over time and with battery life. What is the mechanism of capacity decay?
1. Volume change
During the charging and discharging process of the battery, lithium ions are embedded in and out, causing the lattices of the positive and negative electrode materials to expand/contract to varying degrees.
A. The microstructure of the cathode material changes, resulting in a decrease in the amount of lithium inserted. Under overcharge conditions, lithium ions rapidly migrate to the anode, causing the cathode lattice to collapse.
B. The volume change of negative electrode graphite during the insertion and extraction of lithium ions can reach 10%, resulting in particle stratification.
2. Formation of SEI film
During the battery formation phase, lithium ions react chemically with certain components of the electrolyte during the initial charge and discharge process, forming an irreversible solid electrolyte interface at the interface between the negative electrode and the electrolyte. During the charge and discharge process, the SEI continuously breaks down and regenerates, resulting in a decrease in active lithium ions, an increase in SEI film thickness, and an increase in internal resistance.
3. Lithium dendrite growth
Under low temperature, fast charging and overcharging conditions, lithium ions continue to move toward the negative electrode. The rate of lithium ion extraction is greater than the rate of lithium ion embedding, resulting in the deposition of lithium ions near the negative electrode and a reduction in active lithium.
4. Electrolyte decomposition
Electrolyte decomposition primarily occurs through two pathways: electrochemical decomposition and chemical decomposition. Electrochemical decomposition is divided into oxidative decomposition on the positive electrode side and reductive decomposition on the negative electrode side. Oxidative decomposition on the positive electrode side occurs when the positive electrode potential is >4.5V, causing battery bulging and increased interfacial impedance. Reductive decomposition on the negative electrode side occurs when the graphite negative electrode potential is <0.8V, causing the battery's SEI to thicken and reducing active lithium. Chemical decomposition is divided into trace water-catalyzed reactions and high-temperature decomposition reactions. Trace water-catalyzed reactions cause positive electrode corrosion. High-temperature decomposition reactions cause the electrolyte to dry out, leading to a tendency toward thermal runaway.
5. Current collector corrosion
Current collector corrosion is categorized as corrosion of the positive electrode aluminum foil at high potential and corrosion of the negative electrode copper foil at low potential. When the positive electrode potential exceeds 3.8V, the positive electrode aluminum foil oxidizes and corrodes. Under overcharge conditions, when the negative electrode potential is less than 3V, the copper foil dissolves, migrates to the positive electrode, and deposits on the positive electrode surface.
There are also failure of conductive agents and aging of diaphragms.


