News
How to Reduce Internal Resistance in Li-ion Batteries: A Practical Guide
Factors affecting battery internal resistance include ionic resistance, electronic resistance and contact resistance:
1.Ionic resistance:
electrolyte conductivity, electrode porosity, diaphragm porosity, etc.;
(1)Improper electrolyte formulation (e.g., too low a lithium salt concentration, an unreasonable solvent ratio) or increased viscosity at low temperatures can reduce ion migration rates. Too little electrolyte can also lead to poor contact between the active material and the electrolyte, increasing internal resistance.
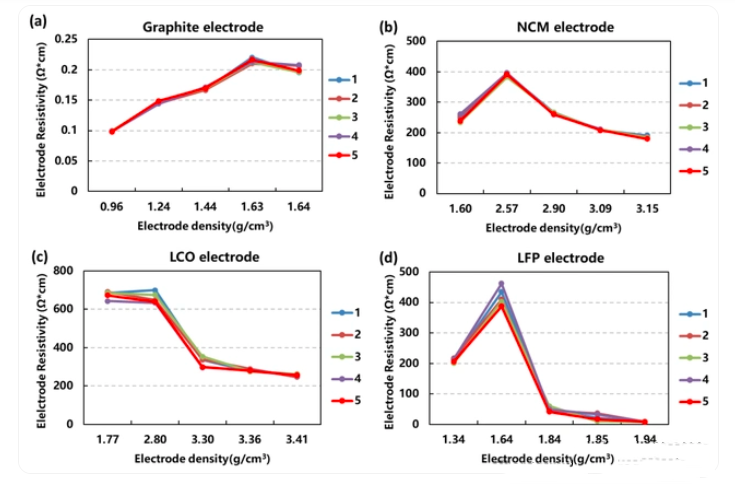
(2)The electrode compaction density is too high. Excessive compaction reduces electrode porosity and restricts electrolyte infiltration. ( Whether the electrode is over-compacted can be determined by observing whether the electrode is brittle, using an electron microscope to check whether the material is broken, and estimating the electrode porosity. The electrode porosity is an important indicator for determining the amount and rate of liquid absorption by the electrode, which has a direct impact on battery performance. )
(3)Low diaphragm porosity or excessive thickness can increase resistance to lithium ion migration. Diaphragm contamination or aging, impurities clogging the pores, or high temperatures causing the diaphragm to shrink/melt, can hinder ion transport. ( Diaphragm porosity is an important indicator of diaphragm physical properties testing .)
2. Electronic resistance:
electrode resistivity, current collector thickness, etc.;
(1)The positive/negative electrode materials have poor conductivity. For example, the intrinsic conductivity of lithium iron phosphate (LiFePO₄) positive electrode material is low. If it is not fully coated with carbon or doped and modified, the electron transfer resistance will increase.
(2)Excessive particle size of the electrode material will extend the lithium ion diffusion path; insufficient porosity will hinder electrolyte infiltration and increase ion migration resistance.
(3)Insufficient or unevenly dispersed conductive agents (such as carbon black) lead to an imperfect electron conduction network within the electrode. The aforementioned factors, including material quality, compaction density, conductive agent dosage, and current collector selection, ultimately manifest themselves in the electrode sheet. Lithium battery companies typically test electrode sheet resistance to determine internal resistance.
3. Contact resistance:
welding between active material and current collector, and between current collector and tab.
(1)The contact internal resistance between the active material and the current collector is large, and carbon-coated copper-aluminum foil can generally be used to increase conductivity.
(2)The welding between the tab and the current collector (such as aluminum foil/copper foil) is not strong, which increases the contact resistance.
(3)The internal pressure of the battery cell is too low (poor contact) or too high (diaphragm deformation), which will affect the internal resistance. The reasons for the high internal resistance of lithium batteries involve many aspects such as materials, manufacturing process, usage conditions and aging.
4. How to reduce internal resistance?
You can consider the following aspects:
(1)Optimize materials: select highly conductive electrode materials and rationally design the pore structure.
(2)Improve the process: ensure uniform electrode coating, control compaction density, and optimize welding quality.
(3)Adjust the electrolyte: Use a high conductivity formula that is suitable for a wide temperature range.
(4)Avoid abuse: Prevent overcharge/overdischarge, high-temperature storage, and control reasonable charge and discharge rates.


